Global Therapeutic Ultrasound Company Submits Application for HIFU System
By MedImaging International staff writers
Posted on 27 Apr 2016
A global leader in therapeutic ultrasound systems has announced the submission of a 510(k) application for the clearance of its next generation non-invasive HIFU device to the US Food and Drug Administration (FDA).Posted on 27 Apr 2016
The company received US FDA clearance in 2015 for another robotic High-Intensity Focused Ultrasound (HIFU) prostate tissue ablation system. HIFU treatment is minimally invasive, and is an effective treatment option for prostatic tissue ablation. The treatment has minimal side effects, and is normally recommended for treatment of localized stage T1 and T2 prostate cancer.
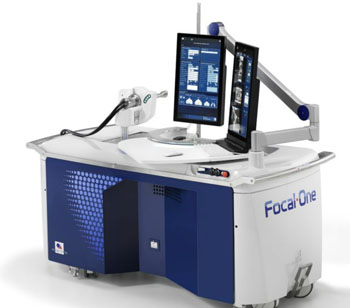
Image: The robot-assisted Focal One HIFU device (Photo courtesy of EDAP TMS).
The new robot-assisted Focal One HIFU device, the EDAP TMS (Paris, France) Focal One system, is intended for focal therapy of prostate cancer. The system already has the CE Mark (Conformité Européenne) for the European market.
The non-invasive Focal One robot-assisted prostate tumorectomy device combines imaging and treatment technologies. The device provides focal therapy, Magnetic Resonance (MR)-fused imaging, precise and efficient therapeutic HIFU energy, and validation imaging using contrast-enhanced ultrasound when treatment is complete.
Marc Oczachowski, CEO of EDAP, said, “We are pleased to submit our Focal One HIFU device file to the FDA to further our goal of making EDAP's full range of HIFU products available to both urologists and patients in the U.S. There is a clear, growing demand from the worldwide urology community for non-invasive options for the ablation of prostatic tissue, and we are well positioned to address this market with our complementary Ablatherm and Focal One devices. We believe that HIFU has the potential to become a standard of care tool for prostate ablation. We are extremely excited by the progress of the U.S. commercial launch of Ablatherm Robotic HIFU and look forward to working with the FDA on the clearance process for Focal One."
Related Links:
EDAP TMS