Ultrasound System Receives US Defense Health Agency’s Approval for Ensuring Protection of Patient Health Information
By MedImaging International staff writers
Posted on 21 Apr 2016
A leading manufacturer of ultrasound systems has received Authorization to Operate (ATO) approval from the US Defense Health Agency (DHA) by complying with strict US Department of Defence (DoD) cybersecurity guidelines.Posted on 21 Apr 2016
The approved series of ultrasound systems met the DHA’s Patient Health Information (PHI) network security guidelines, which mitigate security risks and ensure protection against viruses, malware, and other malicious software. To meet the PHI guidelines, the manufacturer enhanced their ultrasound platform to include the Microsoft Windows 7 Operating System (OS) and security software that proactively identifies and prevents malicious attacks and viruses. Cybersecurity is also an increasing problem for healthcare institutions outside of the military that are increasingly connected on online networks.
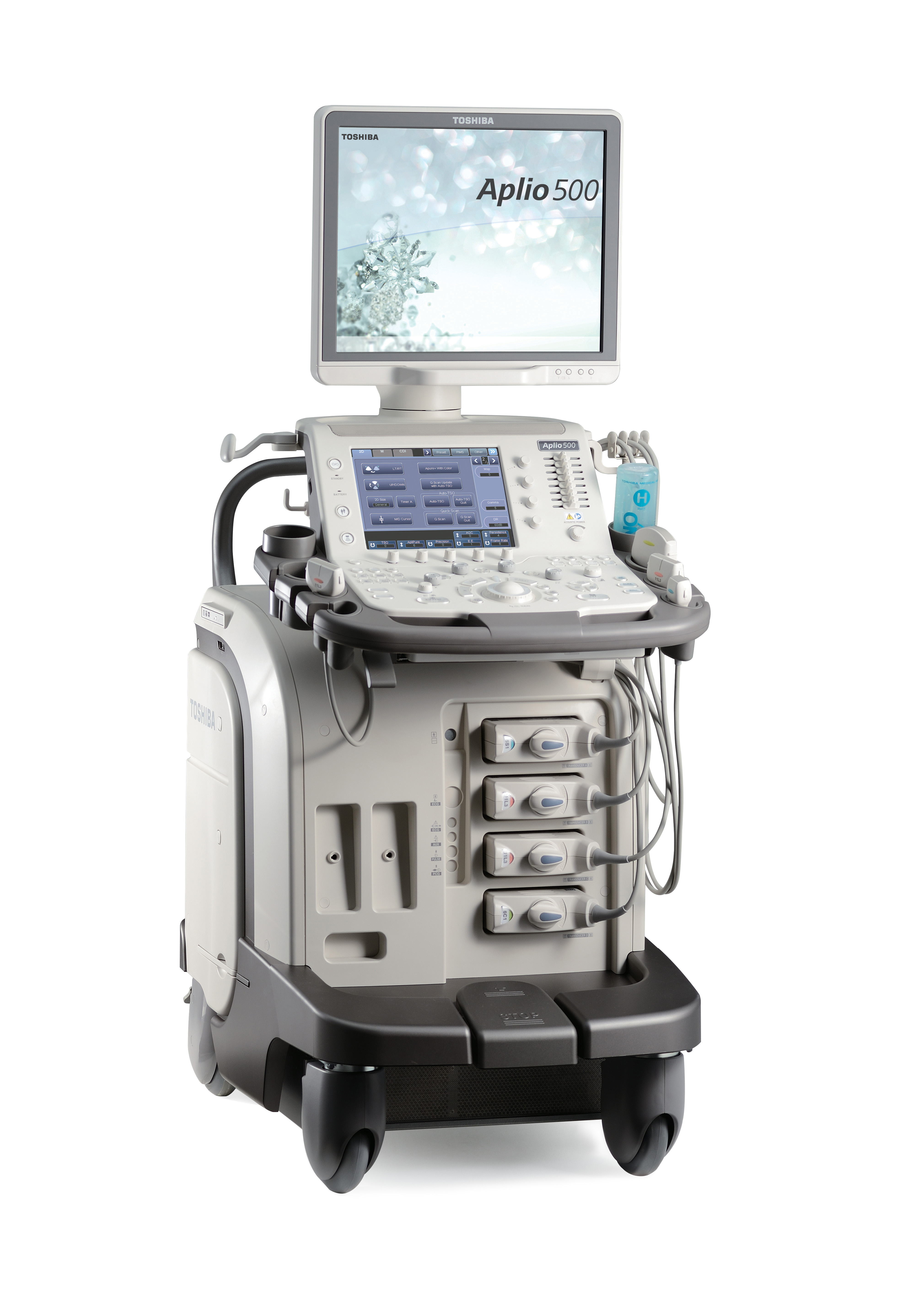
Image: The Aplio 500 Platinum ultrasound system (Photo courtesy of Toshiba Medical Systems).
The ultrasound systems that were approved were the Aplio 500 Platinum and Aplio 300 Platinum series. The systems are manufactured by Toshiba America Medical Systems (Tustin, CA, USA), and received ATO approval for use in the US Air Force. In the past Toshiba also received ATO approval for its Computed Tomography (CT), Magnetic Resonance (MR), and cardiovascular X-Ray systems.
Satrajit Misra, VP, Marketing and Strategic Development, at Toshiba, said, “We understand that medical imaging safety goes beyond radiation dose and extends to other areas, including cyber threats where medical imaging technology could be targeted. Cyber threats present a significant risk to not only healthcare provider’s data, but to patient safety when personal health and financial information is stolen, and the confirmation of ATO status will help our customers mitigate those risks.”
Related Links:
Toshiba America Medical Systems