New Cardiac Ultrasound Features 4D Transesophageal Probe
By MedImaging International staff writers
Posted on 07 May 2012
A new cardiac ultrasound system includes a four-dimensional (4D) transducer for transesophageal echocardiography (TEE), and also provides advanced tools designed to improve workflow efficiency through intuitive navigation, simplified image acquisition, and sophisticated yet easy to use quantification. Posted on 07 May 2012
GE Healthcare (Chalfont St. Giles, UK) recently reported that the US Food and Drug Administration (FDA) clearance and availability of the latest version of its Vivid E9 Vivid E9 Breakthrough 2012 (BT12) cardiovascular ultrasound system.
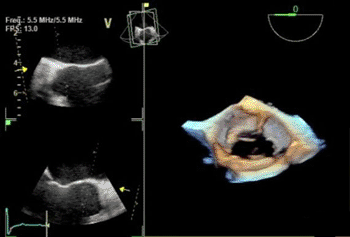
Image: The Vivid E9 Breakthrough 2012 includes a 4-D transducer for transesophageal echocardiography (TEE), as well as tools for workflow efficiencies (Photo courtesy of GE Healthcare).
Whereas Vivid E9 is currently used most frequently in the echo lab, the availability of the 4D TEE transducer enables the system to be used more often in environments such as the cath lab and the operating room. The TEE transducer will allow clinicians to view clear images of the heart during evaluation/diagnosis performed in the echo lab, and support invasive surgical procedures in the operating room, as well as image-guided procedures in the cath lab. With the TEE transducer, Vivid E9 can now be utilized for procedures including mitral valve repair, transcatheter aortic valve repair (TAVR/TAVI), atrial septal defect (ASD) closures, and patent foraman ovale (PFO) closures.
“The new 4D transesophageal probe developed by GE Healthcare is an advanced diagnostic tool,” said Dr. Luigi P. Badano, from the University of Padua (Padua, Italy). “It combines ease of use and flexibility to acquire 4D data sets, user-friendly navigation tools that allow users to quickly visualize the cardiac structure of interest, and effective quantification software to quantitate left ventricular-pump function, myocardial deformation, and mitral valve [MV] morphology.”
The Vivid E9 BT12 also includes several features that are new to cardiovascular ultrasound, designed to enhance 4D imaging and workflow. These include a configurable TEE transducer, a new tilt and rotate function, and a live “2-Click Crop” tool. Workflow enhancements can also be accomplished during mitral valve acquisition with a dedicated MV button and during quantification with a new plug-in tool that has the potential to reduce evaluation time.
Related Links:
GE Healthcare