Mini Access System Supports Precise Needle Placement
By MedImaging International staff writers
Posted on 16 Apr 2012
A sextant-like device for central venous catheter (CVC) placement provides a depth scale to adjust positioning, and fits standard ultrasound (US) transducers for observing needle bearing.Posted on 16 Apr 2012
The Euclid Tier 1 Mini Access System is designed to access and/or place a guidewire into a blood vessel located 5-60 mm below the skin surface. The system is portable, hand operated, and used in conjunction with commercially available US imaging systems. When coupled, the clinician has the ability to accurately locate, determine the depth, and insert a needle and guidewire into the selected vessel; US imaging enables the user to determine whether or not the guidewire is placed correctly within the vessel.
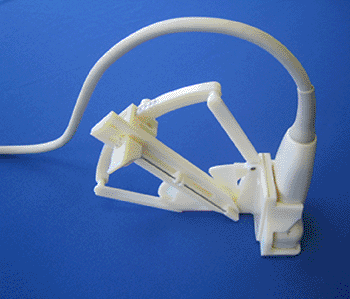
Image: The Euclid Tier 1 Mini Access System (Photo courtesy of Houston Medical Robotics).
Technology limitations, inadequate procedure training and exposure, and varying patient size and morphology have all been identified as co-contributors to the high CVC placement complication rates. Use of US imaging during such procedures has been shown to reduce complications, but is often cumbersome and generally requires additional assistance. The Euclid Tier 1 Mini Access System, specifically designed to overcome these issues, is a product of Houston Medical Robotics (TX, USA), and has been approved by the US Food and Drug Administration (FDA).
“Although numerous applications have been identified, our initial target for the Euclid Tier 1 Mini Access System is reducing complications associated with central venous catheter placement,” said Jeffery J. Sheldon, chairman and CEO of Houston Medical Robotics. “With over 5 million CVCs placed each year - and published complication rates are as high as 26% - we are poised to make a significant impact on healthcare outcomes.”
CVC’s are generally placed into the larger veins, such as the internal jugular vein, the subclavian vein, the axillary vein, or the femoral vein. It is used to administer medication or fluids, obtain blood tests (specifically mixed venous oxygen saturation), and directly obtain cardiovascular measurements such as the central venous pressure (CVP). Other, long-term indications include dialysis, administration of antibiotics, parenteral nutrition (especially in the chronically ill), and chemotherapy. Complications include pneumothorax, central-line associated bloodstream infections (CLABSIs), thrombosis, and hemorrhage.
Related Links:
Houston Medical Robotics