Samsung Ushers in New Era of Diagnostic Solutions
Posted on 27 Nov 2023
NeuroLogica Corp., a subsidiary of Samsung Electronics (Suwon-si, South Korea), is showcasing its latest offerings in mobile computed tomography (CT) and featuring the OmniTom Elite Photon Counting mobile CT scanner at the Radiological Society of North America (RSNA) Annual Meeting.
The clinical launch of the OmniTom Elite with PCD, the first US FDA 510(k) cleared single-source photon counting CT scanner with a single detector on a mobile system, is being featured at this year’s meeting. Photon counting is a next-generation CT technology that sorts the different energies of X-rays after they have passed through the scan field, capturing the X-ray photons directly at the detector and converting the energy to electrical signals. The OmniTom Elite’s ability to provide versatile, real-time mobile imaging enables healthcare providers to administer point-of-care CT to critical patients without the need to transport them to a separate department, expanding the diagnostic possibilities of CT at the patient’s bedside.
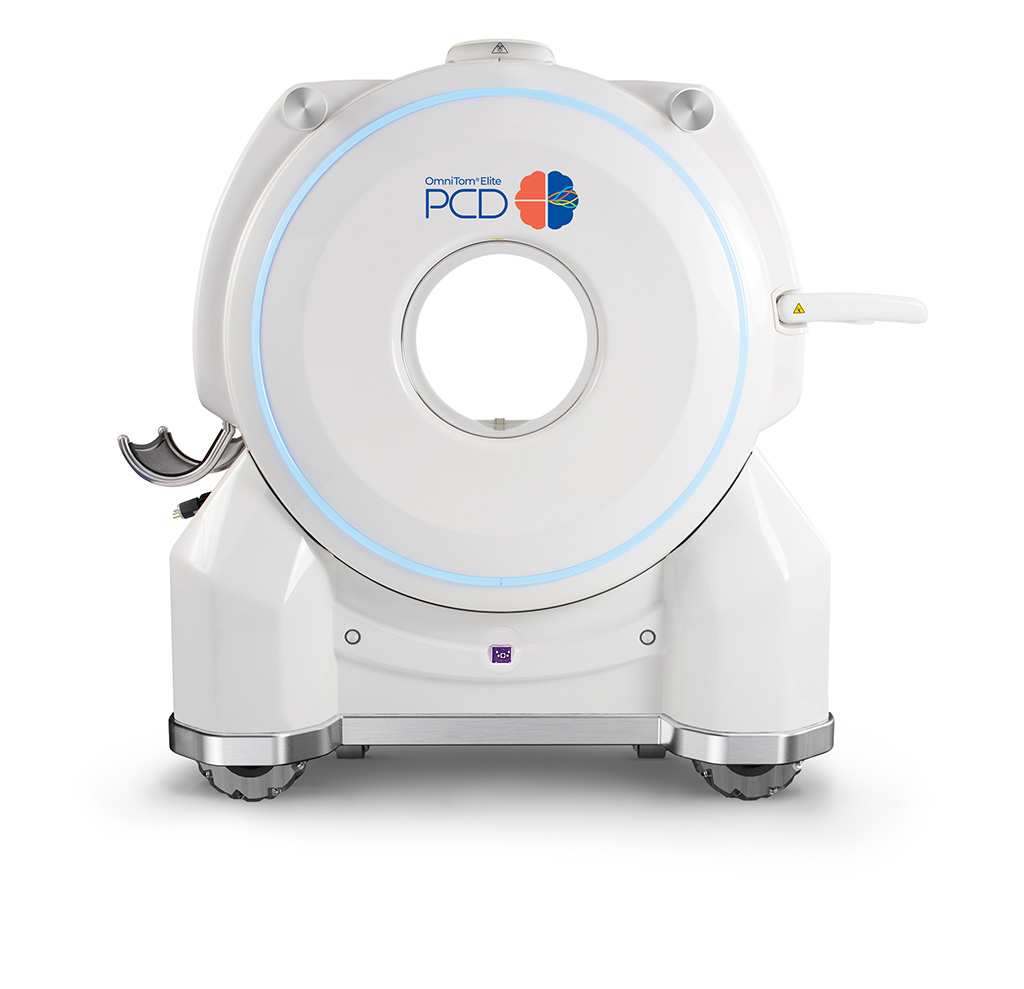
The OmniTom Elite mobile CT scanners were designed keeping in mind upgrading capabilities. In the near term, all current OmniTom users will have the opportunity to upgrade their scanners with the PCD technology. NeuroLogica has built on design features from the first US FDA US 510(k) clearance in 2022 to the second one in June 2023 to include high-resolution mode scanning and multi-energy CT functionality with spectral capability for material decomposition and virtual monoenergetic images (VMI). NeuroLogica is unveiling the OmniTom Elite with PCD and offering a live demonstration of this state-of-the-art technology at Booth #6113. In addition, NeuroLogica is hosting the Samsung NeuroLogica Lunch & Learn Event “Photon Counting: The Next Generation of Neuro CT” where industry experts will discuss how leveraging the latest innovation in CT applications can increase productivity and enhance the patient and physician experience.
“The OmniTom Elite is an advanced imaging solution that is extremely mobile, giving healthcare providers more flexibility in when and where they run diagnostic tests for their patients,” said Renaud Maloberti, Head of Business, MCT. “We look forward to featuring this innovative technology at this year’s Radiological Society of North America Annual Meeting.”
Related Links:
Samsung Electronics