FDA Clearance Announced for Ceiling-Mounted Digital Radiography System
By MedImaging International staff writers
Posted on 08 Sep 2015
The US medical imaging subsidiary of a global electronics manufacturer has announced US Food and Drug Administration (FDA; Silver Spring, MD USA) 510(k) clearance of its newest Digital Radiography (DR) system.Posted on 08 Sep 2015
The new system is the latest in a line of DR systems from the same manufacturer and boasts advanced imaging technology and improved user-friendly workflows, together with a reduced total cost of ownership. The system’s energy saving mode uses half the amount of power compared to ready to scan mode, and the system minimizes excessive radiation exposure by means of an automatic sensor, auto exposure control, and provides X-ray dose information measured using the Dose Area Product (DAP).

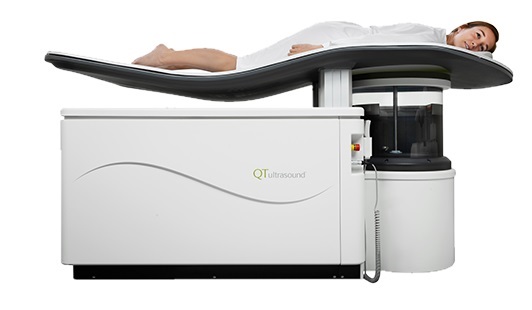
Image: Samsung GC85A Ceiling-mounted Digital Radiography System (Photo courtesy of Samsung).
Key features of the Samsung GC85A ceiling-mounted DR system, developed by NeuroLogica (Danvers, MA, USA), a Samsung Electronics America (Ridgefield Park, NJ, USA) subsidiary, include the S-Vue imaging engine, one-touch positioning, S-Align, Smart Stitching, and the wireless lightweight S-Detector with high Detective Quantum Efficiency (DQE) and a portable grid that can display the patient’s body structure without increasing radiation dose. The S-Detectors are compatible with other Samsung DR systems, and in case of component failure, ceiling DR S-Detectors can be used with other systems.
David Webster, vice president, Global Sales and CMO at NeuroLogica, said, “The Samsung GC85A represents NeuroLogica’s latest commitment to introducing user- and patient-centric innovation to healthcare to provide fast, easy and accurate diagnoses. The system’s superior image quality and ease of control will enable users to experience a new level of efficiency with a DR system designed for streamlined operation.”
Related Links:
NeuroLogica
Samsung Electronics America