Noninvasive Test Approved for the Evaluation of Coronary Artery Disease
By MedImaging International staff writers
Posted on 14 Jan 2015
The US Food and Drug Administration (FDA) have allowed marketing of the HeartFlow (Redwood City, CA, USA) FFR-CT software.Posted on 14 Jan 2015
Normally Fractional Flow Reserve (FFR) data is obtained from an invasive cardiac catheterization procedure. The HeartFlow application uses data from a standard Computed Tomography (CT) scan and enables healthcare professionals to measure ischemia, and create a 3-D model of the blood flow in the coronary arteries of a patient with symptoms of coronary heart disease. HeartFlow provides noninvasive evaluation of blockages in coronary arteries using computational fluid dynamics and can help evaluate the effectiveness of placing a stent, angioplasty, or surgery.
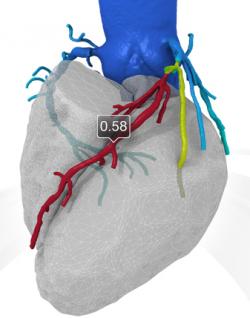
Image: 3-D Representation of the Heart and Coronary Arteries (Photo courtesy of HeartFlow).
In tests HeartFlow FFR-CT was found to be 84%-86% accurate in differentiating significant coronary artery blockages that need revascularization from those that do not, and provides a fast, noninvasive method for the diagnosis of coronary heart disease.
William Maisel, MD, MPH, deputy director for science and chief scientist in the FDA’s Center for Devices and Radiological Health stated, “This noninvasive method is an additional tool for clinicians who are considering the risks and benefits of invasive coronary procedures.”
Related Links:
HeartFlow














