Shock-Absorbing “Goo” Discovered in Bone
By MedImaging International staff writers
Posted on 09 Apr 2014
A combination of imaging techniques and computational modeling reveals that much of the mineral from which bone is made consists of “goo” trapped between tiny crystals that provides a flexibility that stops bones from shattering.Posted on 09 Apr 2014
Researchers at the University of Cambridge (United Kingdom) and University College London (UCL; United Kingdom) uses a combination of multinuclear solid-state nuclear magnetic resonance (NMR), spectroscopy, powder X-ray diffraction, and first principles electronic structure calculations to propose a quantitative structure for double salt octacalcium phosphate citrate (OCP-citrate) bridging between layers of calcium phosphate mineral (such as in bone), in which citrate anions reside in a hydrated layer, bridging between the apatitic layers.
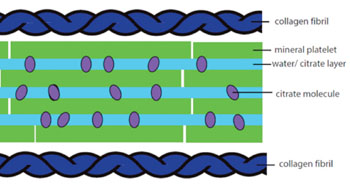
Image: The newly proposed layered structure of bone minerals (Photo courtesy of Cambridge University).
According to the model, citrate—a by-product of natural cell metabolism—is mixed with water to create a viscous fluid that is trapped between the nanoscale calcium phosphate crystals that form bones. This fluid allows enough movement (slippage), between the crystals so that bones remain flexible, and do not shatter under pressure. Bone tissue also has a protein mesh with holes where the calcium is deposited. In healthy tissue, the holes are very small, so that when the calcium is deposited, the citrate cannot escape and is trapped between crystals, creating the flexible layers of fluid and bone plates.
But as people age or suffer repeated bone trauma, the protein mesh becomes irreparably damaged, resulting in progressively larger holes that allow the citrate fluid to leak out. The calcium phosphate crystals can then fuse together into bigger and bigger clumps unimpeded by citrate, turning the bone inflexible, increasingly brittle, and more likely to shatter. According to the researchers the model can explain a number of known structural features of bone mineral, such the thin, plate-like morphology of mature bone mineral crystals and the presence of strongly bound water molecules, as well explain the root cause of osteoporosis. The study was published early online on March 21, 2014, in the journal Proceedings of the National Academy of Sciences of the United States of America (PNAS).
“What we've shown is that a large part of bone mineral – possibly as much as half of it in fact – is made up of this goo, where citrate is binding like a gel between mineral crystals,” said lead author Melinda Duer, PhD, of the Cambridge department of chemistry and advanced imaging center. “The crystals stay in flat, plate-like shapes that have the facility to slide with respect to each other. Without citrate, all crystals in bone mineral would collapse together and become one big crystal and shatter.”
Related Links:
University of Cambridge
University College London














