Single Room System Advances Proton Therapy Accessibility
By MedImaging International staff writers
Posted on 17 Oct 2019
A compact proton therapy (PT) system equipped with advanced pencil beam scanning and integrated imaging and control systems expand the accessibility of PT.Posted on 17 Oct 2019
The ProTom International (Wakefield, MA, USA) Radiance 330 proton therapy system boasts a compact synchrotron that serves as the source of the proton beam, composed of an injector that generates the proton beam and the synchrotron, which accumulates, accelerates, and extracts the proton beam. Depending on the configuration required, the transport and delivery sub-systems control the guidance, irradiation dose, and shape of the proton beam, and direct the beam appropriately. The Radiance 330 is installed at Massachusetts General Hospital (MGH; Boston, USA).
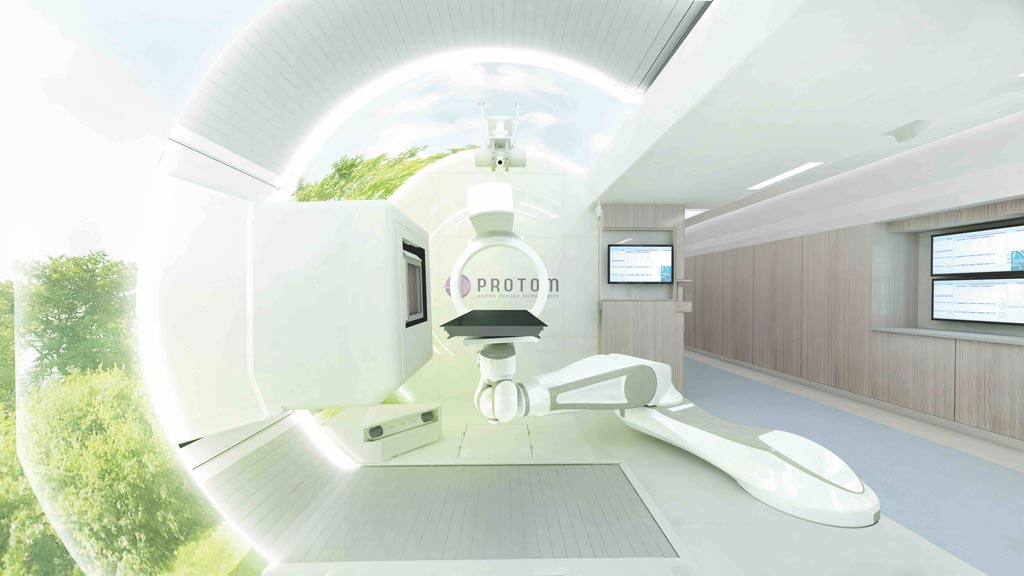
Image: The Radiance330 proton therapy system treatment room (Photo courtesy of ProTom International).
The Radiance 330 is designed to interface with a range of in-room imaging solutions, such as computed tomography (CT) or cone-beam computed tomography (CBCT), and its 330 MeV capacity also allows for proton imaging of any anatomical area. An additional feature is orthogonal imaging, including image-registration software, which generates a six-degree of freedom patient-alignment correction vector. The compact system can be installed within an interior accelerator vault space of just 6 x 9 meters, and requires up to 40% less radiation shielding.
“ProTom is a company devoted to proton therapy as its sole mission; this is all we do. We thank our customers, partners, and suppliers for their confidence and support throughout this process,” said Steve Spotts, CEO and co-founder of ProTom International, upon receipt of U.S. Food and Drug Administration (FDA) clearance. “This achievement accelerates ProTom's single and relentless mission to place this highly sophisticated and targeted cancer-fighting tool within reach of many more physicians.”
Photon radiation typically uses multiple X-ray beams to attack a tumor target, but unavoidably deposits radiation in the normal tissues beyond the target, potentially damaging those tissues as the beam exits the body. Proton therapy, an alternative radiation, works differently, by directing positively charged protons at the tumor target, where they deposit the bulk of the radiation dose, with minimal residual radiation delivered beyond the target, potentially reducing side effects and damage to surrounding tissue.
Related Links:
ProTom International














