Consumer Advocacy Agency Advises MRI Scanning Needed for All-Metal Hip Implant Recipients
By MedImaging International staff writers
Posted on 22 May 2014
A magnetic resonance imaging (MRI) scan can help detect premature failure of all-metal hip implants before symptoms appear, according to a US consumer advocacy agency.Posted on 22 May 2014
The US Drug Watchdog (Washington DC, USA), a consumer advocate agency created for victims of recalled drugs, is expressing concern for recipients of an all-metal hip implant, and premature failures that could cause additional medical issues such as metallosis.
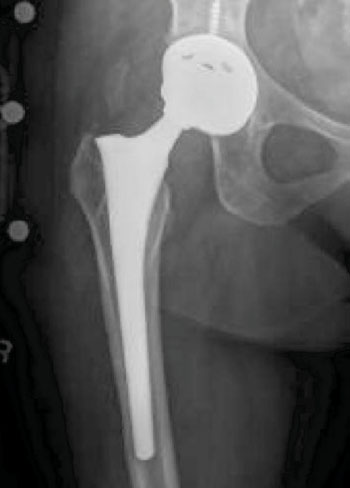
Image: Metal-on-metal (MoM) hip implant (Photo courtesy of the US Drug Watchdog).
The US Drug Watchdog’s spokesperson reported, “We do not want any recipient of an all-metal hip implant to get left behind with no settlement if their all-metal hip implant has prematurely failed….all individuals who now have a cobalt and chromium hip [should] get an MRI for conclusive proof of the condition of their hip implant. If there is tissue inflammation in the area of the stem, or the ball, and cup of the hip implant it is a very good indication of a premature failure.”
Metal-on-metal (MoM) hip implants can cause inflammation of the joint lining (synovitis) long before symptoms appear, and MRI can be used to identify this inflammation, according to a new study by researchers from the Hospital for Special Surgery (New York, NY, USA). The study that appears in an upcoming issue of the Journal of Bone & Joint Surgery demonstrates that MRI can be used to identify implants that are going to fail before people become symptomatic.
The goal of the US Drug Watchdog is to inform US consumers about lethal US Food and Drug Administration (FDA) drug recalls, and recalled defective medical devices.
Related Links:
Hospital for Special Surgery
US Drug Watchdog