Ultrahigh-Field MRI Shows Potential for Earlier Diagnosis of Parkinson’s Disease
By MedImaging International staff writers
Posted on 20 Mar 2014
New research has revealed that ultrahigh-field magnetic resonance imaging (MRI) provides detailed views of a brain area implicated in Parkinson’s disease (PD); possibly leading to earlier detection of a disorder that affects millions worldwide.
The study’s findings are published online March 2014 in the journal
Radiology. Parkinson’s disease is a chronic, progressive disease characterized by stiffness, shaking, and impaired balance and coordination. With no imaging modalities available to help in diagnosis, clinicians have had to depend on medical history and neurologic exam. It is frequently problematic to distinguish PD from other disorders using these techniques alone.
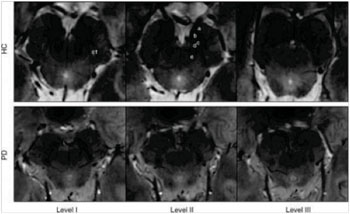
Top row: 7-Tesla 3D multiecho susceptibility-weighted in vivo images of SN in healthy 64-year-old man, located between the crus cerebri (a) and the red nucleus. Axial sections perpendicular to the floor of fourth ventricle are obtained at level of the inferior third of the red nucleus (level I), at the level of decussation of superior cerebellar peduncles (e) (level II), and at the level of the inferior colliculi (level III). At level I, substantia nigra (SN) appears as homogeneous hypointense structure in the medial part of the cerebral peduncle, and is laterally constituted by a hyperintense oval area between two hypointense layers (c1). At level II, a trilaminar organization of the SN with a central hyperintense layer (b) between two hypointense tiers (c and d) is detectable. At level III, the dorsal hypointense lamina could be detected as a small residual lateral hypointense area, while the hyperintense layer fades into the isointense cerebral peduncle. Bottom row: 7-T three-dimensional multiecho susceptibility-weighted in vivo images of the SN in PD patients. The loss of normal anatomy of the SN in a 61-year-old man with PD is characterized by the disappearance of the oval-shape bright spot in the lateral part of the SN at level I and by the loss of the hyperintense intermediate layer of the SN at level II. HC = healthy subject (Photo courtesy of the Radiological Society of North America).
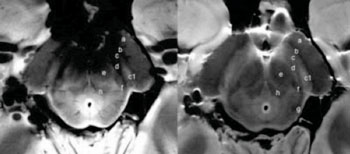
Image: axial spin-echo proton density (on the right) and GRE (on the left) of the substantia nigra (SN) at level I of an ex vivo brain sample in a 67-year-old woman. There is a triple-layered organization of the SN comparable to that showed in the in vivo images. Ventrally a low-signal-intensity layer (b) is attributable to the pars reticulata of the SN. In the middle part of the SN, a hyperintense band (c) corresponds to the ventral component of the pars compacta of the SN. The lateral part of this layer shows a high-signal intensity spot (c1) corresponding to the oval shape hyperintensity of the in vivo 3D multiecho susceptibility-weighted images that resemble the nigrosome formation. The dorsal hypointense layer visible on both spin-echo and GRE images (d) is referred to the dorsal component of the pars compacta of the SN. a = crus cerebri, e = brachjum conjunctivum, f = medial lemniscus, g = lateral lemniscus, h = central tegmental tract (Photo courtesy of the Radiological Society of North America).
Mirco Cosottini, MD, from the University of Pisa (Italy), and colleagues examined the brains of 38 individuals, including 17 PD patients and 21 healthy control subjects, as well as a brain sample from a deceased individual, to help determine the effectiveness of ultra-high-field 7-Tesla MRI for identifying PD.
The investigators, utilizing the 7-Tesla MRI scanning, were able to differentiate a three-layered organization of the substantia nigra (SN), a crescent-shaped mass of cells in the midbrain. PD results from the loss of dopamine-producing cells located in this region of the brain. Dopamine is an important neurotransmitter involved in a number of brain functions, including motor and behavioral processes such as addiction, mood, reward, and stress.
Based on abnormalities in the SN identified by the 7-Tesla MRI scanning, the researchers accurately categorized patients with PD with a sensitivity of 100% and specificity of 96.2%.
According to Dr. Cosottini, the findings show potential for earlier detection of the disease, which could speed the initiation of treatment. “Parkinson’s disease diagnosis remains clinically based, but with the introduction of 7-Tesla MRI into clinical practice, a supporting radiologic diagnosis can be made,” he said.
The researchers also are investigating the clinical usefulness of 7-Tesla MRI in several other neurodegenerative disorders, including mild cognitive impairment, a precursor of Alzheimer’s disease.
Related Links:
University of Pisa















.jpg)