Implantable Defibrillator Series MRI Compatible
By MedImaging International staff writers
Posted on 15 Apr 2013
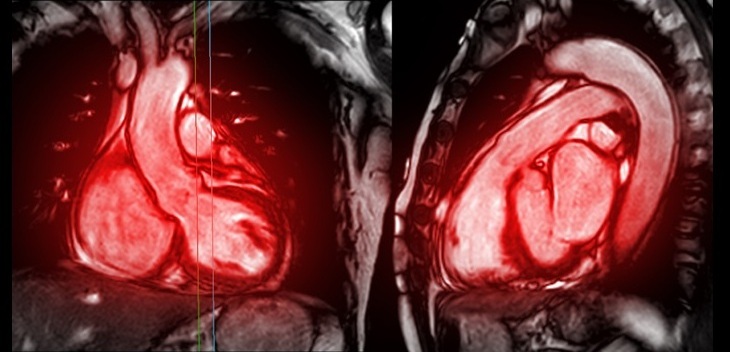
An advanced cardioverter-defibrillator/cardiac resynchronization therapy defibrillator (ICD/CRT-D) series can be used during magnetic resonance imaging (MRI) procedures.Posted on 15 Apr 2013
The Iforia series of ICD/CRT-D devices feature ProMRI technology, which enables access to potentially life-saving MR scans when needed. The Iforia series also includes the industry standard DF4 connector system, designed to simplify and shorten the implantation procedure by combining the traditional three connectors into one, allowing for a more convenient implantation procedure without compromising safety. It also reduces the material in the insertion pocket, the risk of lead-to-port mismatch, and may also lower the risk of lead abrasion.

Image: The Iforia 7 VR-T ICD/CRT-D device (Photo courtesy of BIOTRONIK).
The Iforia series also offer several of the smallest ICDs and CRT-Ds on the market, as well as marked longevity, with the Iforia single chamber ICD reaching more than 11 years, while the Iforia CRT-D has a longevity of more than 7 years. This allows for a low replacement rate, offering clear benefits for both patients and the health care system. The series also includes secure remote monitoring via a Home Monitoring feature. The Iforia series of ICD/CRT-D devices are products of BIOTRONIK (Berlin, Germany), and has received the European community CE marking of approval.
“BIOTRONIK has consistently driven innovation since its foundation in 1963. In the past 15 months alone, BIOTRONIK received market approval for two series of ICDs and CRT-Ds, both of which enable patients to have access to MR scans. The new Iforia series, combined with ProMRI and BIOTRONIK Home Monitoring, offers physicians and patients the best combination of reliability and innovation,” said Christoph Böhmer, President International of BIOTRONIK.
“With the Iforia series I can provide my patients with a small and highly reliable ICD and CRT-D series. It includes an impressive range of state-of-the-art innovations including ProMRI and home monitoring,” said Ching Chi Keong, MD, of the National Heart Centre (Singapore). “In addition the DF4 technology simplifies the implantation procedure, while the small size of the device makes it more comfortable for the patient and also leads to a better cosmetic result.”
Related Links:
BIOTRONIK