Linac-Based MRI-Guided Radiation Therapy System Awarded CE Mark
By MedImaging International staff writers
Posted on 26 Sep 2016
The manufacturer of a next-generation linear accelerator, the only clinical MRI-guided radiation therapy system in the world, has announced that they have received CE (Conformité Européenne) mark approval for their new product.Posted on 26 Sep 2016
The Magnetic Resonance Imaging (MRI)-guided radiation therapy system facilitates radiation therapy treatments, by providing MRI alignment, and real-time MRI tracking. The system provides pre-treatment images and soft-tissue imaging during radiation therapy treatment to enable clinicians to see and follow the tumor target, and radiation delivery, sparing healthy tissue. The system includes built-in treatment planning software, and advanced tools for contouring.
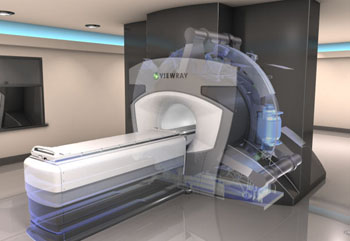
Image: The MRIdian is intended for imaging and treating cancer patients in real-time (Photo courtesy of ViewRay).
ViewRay (Cleveland OH, USA) developed the MRIdian system and has also submitted an application for 510(k) approval of the technology by the US Food and Drug Administration (FDA). The MRIdian is now available for clinical use in Europe, and for use in non-clinical research in the US.
The MRIdian system enables clinicians to observe, assess, and personalize treatments while the radiotherapy beam is on. This enables them to see the target and observe where the radiation is actually being delivered, and adapt to changes in the patient’s anatomy.
President and CEO of ViewRay, Chris A. Raanes, said, "We are excited to have CE Mark approval and to begin selling MRIdian Linac within Europe. We believe the radiation oncology community has been eagerly awaiting the availability of a clinical MRI-guided Linac system, and we're pleased to bring them ViewRay's well-established MRI-guidance capabilities with the familiar functionality of a linac-based platform. We now look forward to progress on our 510(k) filing with the FDA."
Related Links:
ViewRay














