CT System Receives FDA Clearance for AI-Based Image Reconstruction Technology
By MedImaging International staff writers
Posted on 07 Nov 2019
Canon Medical Systems USA, Inc. (Tustin, CA, USA) has received 510(k) clearance for its Advanced Intelligent Clear-IQ Engine (AiCE) for the Aquilion Precision, further expanding access to its new deep convolutional neural network (DCNN) image reconstruction technology. The technology, which is now available on Canon’s Aquilion Precision and Aquilion ONE/GENESIS Edition CT systems, uses a deep learning algorithm to differentiate signal from noise so that it can suppress noise while enhancing signal, thus improving CT image reconstruction.Posted on 07 Nov 2019
Aquilion Precision, the world’s first ultra-high resolution CT, provides two times the resolution of conventional CT, revealing details that are typically only seen in Cath labs. With AiCE, the system now enables clinicians to perform super-high resolution studies at doses equivalent to standard resolution CT (with traditional hybrid iterative reconstruction techniques). AiCE learns from the high image quality of Model Based Iterative Reconstruction (MBIR) to reconstruct CT images with improved high contrast spatial resolution.
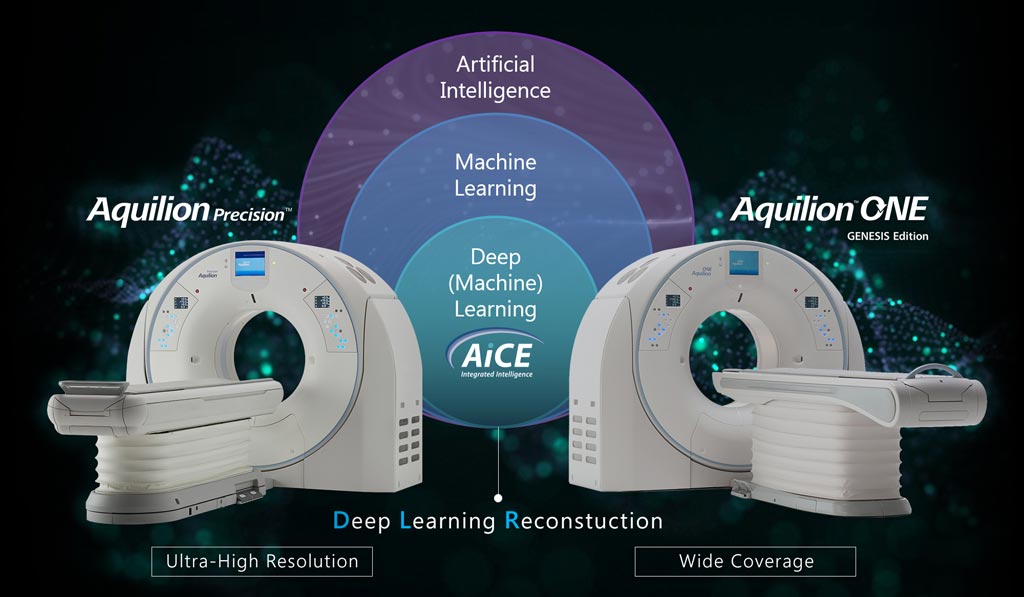
Image: The AiCE technology is intended to enable clinicians to perform super-high resolution studies on the Aquilion Precision CT system (Photo courtesy of Canon Medical Systems).
“As we strive for precision medicine, we realize the importance of starting with the foundation of a precise diagnosis. Canon Medical is committed to developing intelligent solutions that help providers generate clearer, more holistic images to help achieve better outcomes, without increasing dose,” said Tim Nicholson, acting managing director, CT Business Unit, Canon Medical Systems USA. “This technology represents a new era in image reconstruction, which may help provide more possibilities in improving patient care than ever before.”
Related Links:
Canon Medical Systems Corporation








 Guided Devices.jpg)





