AI-Powered Radiology Software Receives FDA Clearance
By MedImaging International staff writers
Posted on 24 Jan 2019
A radiology software product named Quantib Neurodegenerative (ND) that assists radiologists in reading MRI brain scans has received clearance from the US Food and Drug Administration (FDA) for distribution in the U.S. Built by Quantib B.V. (Rotterdam, ZH), a developer of artificial intelligence (AI) tools for radiologists, the software measures brain atrophy (shrinkage) and detects white matter hyperintensities (WMHs), which are changes in the brain related to, for example, aging, dementia and multiple sclerosis (MS).Posted on 24 Jan 2019
Quantib develops AI software for radiologists that uses advanced machine learning techniques to detect changes in tissue sooner than would be possible with the naked eye. The company offers various multiple machine-learning products cleared by the FDA and CE that focus on the quantification and diagnosis of neurodegenerative diseases.
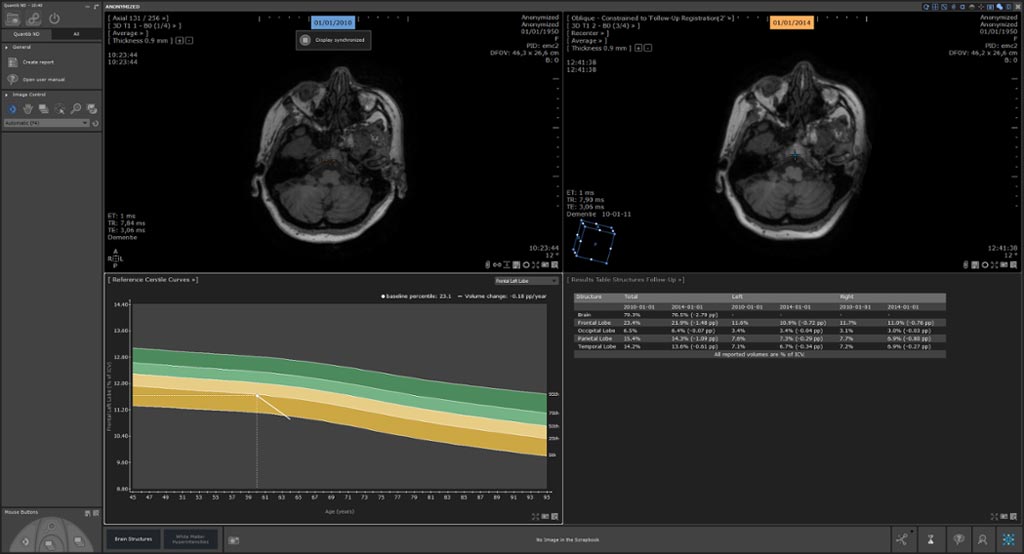
Image: The Quantib Neurodegenerative (ND) software that assists radiologists in reading MRI brain scans has received clearance from the US FDA (Photo courtesy of Quantib).
Quantib ND includes fully automatic segmentation of lobes and hippocampus to objectively assess atrophy development. Additionally, it comprises white matter hyperintensity segmentation for easy monitoring neurological changes occurring in for example dementia and MS patients. Reference centile curves, derived from the population-based Rotterdam Scan Study, provide an intuitive tool to compare the patient’s brain volume to the average of an unbiased population.
The software’s follow-up feature tracks neurodegeneration over time. Accurate alignment of images at subsequent time points enable accurate detection and staging of atrophy, combined with WMH tracking. Quantib ND visualizes new and previously identified lesions using color-coded overlays, while its editing feature provides the radiologist with full control of the final results before sending them to the PACS.
“This is an important achievement,” said Arthur Post Uiterweer, CEO of Quantib. “We are very proud to offer physicians an extensive software package for quantitative measurements of neurological changes that can be related to dementia and MS.”
Related Links:
Quantib