Cancer Detection Technology Built on AI Receives FDA Clearance
By MedImaging International staff writers
Posted on 25 Dec 2018
A new deep-learning cancer detection software solution for digital breast tomosynthesis (DBT) has received clearance from the US FDA for commercial sales and clinical use in US. The solution named ProFound AI is built on artificial intelligence (AI) and has been developed by iCAD, Inc (Nashua, NH, USA), a provider of cancer detection and therapy solutions.Posted on 25 Dec 2018
ProFound AI is a deep-learning cancer detection and workflow solution for DBT that delivers critical benefits to radiologists, their facilities, and their patients through an improvement in cancer detection rates by an average of 8% and reduction in unnecessary patient recall rates by an average of 7%. The technology is trained to detect malignant soft-tissue densities and calcifications. It also provides radiologists with scoring information representing the likelihood that a detection or case is malignant based on the large dataset of clinical images used to train the algorithm.
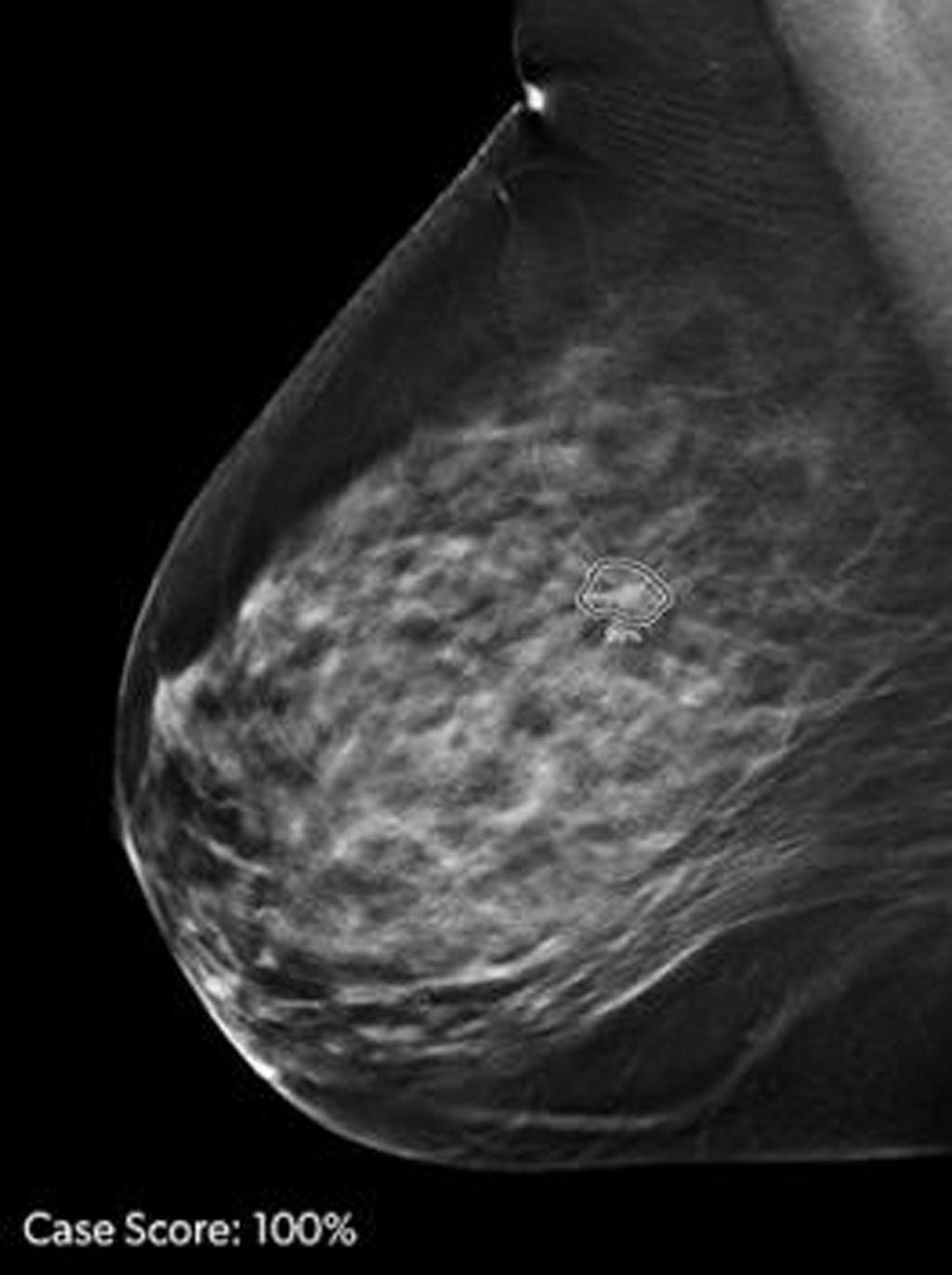
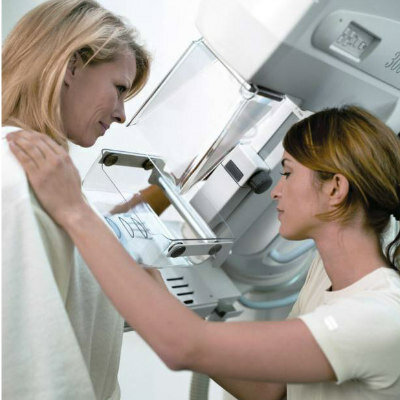
Image: ProFound AI is a deep-learning cancer detection and workflow solution for DBT (Photo courtesy of iCAD).
ProFound AI has received FDA clearance based on positive clinical results from a large reader study completed earlier this year and presented at this year’s Radiological Society of North America (RSNA) annual meeting in Chicago, USA. In the study, 24 radiologists read 260 tomosynthesis cases, both with and without the ProFound AI solution. The study’s findings revealed increased cancer detection rates, reduced false positive rates and patient recalls, and a significant decrease of more than 50% on average in interpretation times.
“Obtaining FDA clearance for ProFound AI opens a new and substantial addressable market for iCAD. This enables us to offer clinicians globally an unrivaled cancer detection and workflow solution built on the latest advances in deep-learning,” said Stacey Stevens, Executive Vice President and Chief Strategy and Commercial Officer at iCAD. “Clinical reader study results and comprehensive stand-alone testing have shown unprecedented improvements in both clinical performance and reading efficiency. We are proud to introduce revolutionary technology that will fundamentally transform breast cancer detection and patient care.”