Stroke Detection Software Receives FDA Clearance
By MedImaging International staff writers
Posted on 21 Feb 2018
The US FDA has approved a new clinical decision support software that notifies neurologists about a potential stroke in their patients by analyzing computed tomography (CT) results. The tool named Contact has been developed by Viz.ai (San Francisco, CA, USA), a direct-to-intervention healthcare company that uses artificial intelligence (AI) and deep learning algorithms to analyze medical data and improve medical workflow.Posted on 21 Feb 2018
The premarket approval of the tool by the FDA was based on a retrospective study of 300 CT images that assessed the independent performance of the image analysis algorithm and notification functionality of the Viz.AI Contact application against the performance of two trained neuro-radiologists for the detection of large vessel blockages in the brain. Real-world evidence was used with a clinical study to demonstrate that the application could notify a neurovascular specialist sooner in cases where a blockage was suspected.
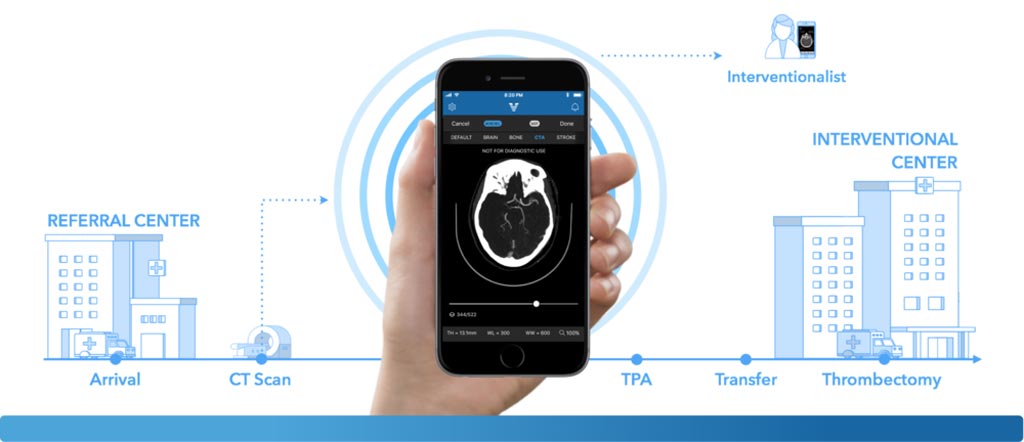
Image: The Viz.AI Contact triage software uses an AI algorithm to analyze images for indicators associated with a stroke (Photo courtesy of Viz.ai).
The Viz.AI Contact application is a computer-aided triage software that uses an AI algorithm to analyze images for indicators associated with a stroke. Designed to analyze CT images of the brain, it sends a text notification to a neurovascular specialist in case it identifies a suspected large vessel blockage. The Viz.AI Contact application automatically notifies the specialist at the same time the first-line provider is conducting a standard review of the images, allowing the specialist sooner to become involved sooner as compared to the usual standard of care wherein patients have to wait for a radiologist to review their CT images and notify a neurovascular specialist.
“Strokes can cause serious and irreversible damage to patients. The software device could benefit patients by notifying a specialist earlier thereby decreasing the time to treatment. Faster treatment may lessen the extent or progression of a stroke,” said Robert Ochs, Ph.D., acting deputy director for radiological health, Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health.
Related Links:
Viz.ai














