New 4D Flow Software Platform Showcased
By Andrew Deutsch
Posted on 07 Dec 2016
A company developing cloud-based medical imaging software has received 510(k) clearance from US FDA for its 4D cardiac flow quantification software.Posted on 07 Dec 2016
The software was showcased at the annual Radiological Society of North America (RSNA 2016) meeting. The company also presented new information about clinical applications that use the 4-D flow software.
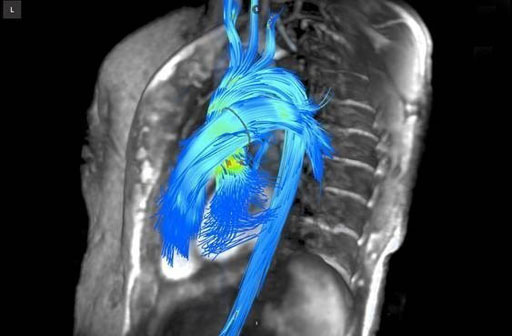
Image: An image generated by the 4D Arterys cardiac flow quantification software (Photo courtesy of Arterys).
The Arterys 4D Flow software was developed by Arterys (San Francisco, CA, USA), a company that develops quantitative imaging tools for supporting treatment decisions. The Arterys 4D Flow platform uses cloud computing and advanced analytics techniques.
The 4D cardiac flow quantification technique uses phase-contrast Cardiac Magnetic Resonance (CMR) 3D data that is velocity-encoded in three spatial directions. The data is resolved relative to the 3 spatial dimensions, and time, for the duration of the cardiac cycle, and enables clinicians to visualize, and measure blood patterns in the 3D volume. The 4D Flow sequence is faster and simpler than conventional MRI scans.
CEO of Arterys, Fabien Beckers, said, "This year, RSNA is focusing a lot of attention on breakthrough technological developments that are moving medical imaging into a new era where it's possible to analyze data to help diagnose conditions much quicker, align various stakeholders in the health ecosystem, and mobilize on personalized treatment paths. We believe the Arterys platform will transform clinical practice through powerful, quantitative imaging tools that rapidly generate and analyze unprecedented amounts of data and insights that can support treatment decisions and the delivery of patient care."
Related Links:
Arterys














