Tomosynthesis Option for Mammography Platform Approved by FDA
By MedImaging International staff writers
Posted on 13 May 2015
The US Food and Drug Administration (FDA; Silver Spring, MD USA) has approved the breast tomosynthesis add-on for Siemens Healthcare (Erlangen, Germany) MAMMOMAT Inspiration digital mammography platforms.Posted on 13 May 2015
The tomosynthesis algorithm creates an approximation of a 3-D image of the breast by reconstructing multiple 2-D breast images. The 3-D image enables detection of tumors that would otherwise be hidden by overlapping breast tissue, reduces false-positive findings, and allows more accurate diagnosis than standard 2-D digital mammography.
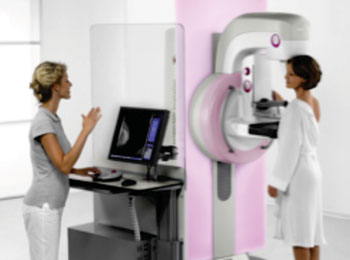
Image: Siemens Healthcare’s MAMMOMAT Inspiration (Photo courtesy of Siemens Healthcare).
The MAMMOMAT acquires 25 two-dimensional images while moving through an angular range of 50 degrees around the breast. The Tomosynthesis algorithm uses the projections to generate a 3-D volume set and reconstructs them as 3-D Digital Breast Tomosynthesis (DBT) images. This helps clinicians assess the size and type of breast lesions and microcalcifications.
Gregory Sorensen, MD, president and CEO of Siemens Healthcare North America, said, “Our clinical data has demonstrated that the addition of Siemens’ digital breast tomosynthesis to a patient’s traditional 2-D digital mammogram increases detection of breast tumors. We know that in clinical practice, this increased diagnostic accuracy also means fewer diagnostic biopsy procedures and fewer anxiety-inducing recalls, which typically contributes to both improved patient outcomes and reduced cost.”
Related Links:
Siemens Healthcare














