Thin Film Electrode Eases Diagnostic Brain Mapping
By MedImaging International staff writers
Posted on 22 Sep 2021
Novel electrode technology provides temporary recording, monitoring, and stimulation of electrical signals at the subsurface level of the brain.Posted on 22 Sep 2021
The NeuroOne Medical Technologies (Eden Prairie, MN, USA) Evo sEEG is a hi-definition thin film electrode (over seven times thinner than a silicone electrode) for short term (up to 24 hours) stereoelectroencephalography (sEEG) monitoring. It is intended for placement on the subsurface level of the brain, without requiring removal of the top section of the patient's skull. The electrode’s polyimide substrate also provides increased flexibility and reduced volume, resulting in increased signal clarity with fewer noise and artifacts, and reduced pain, edema, and immunological response.
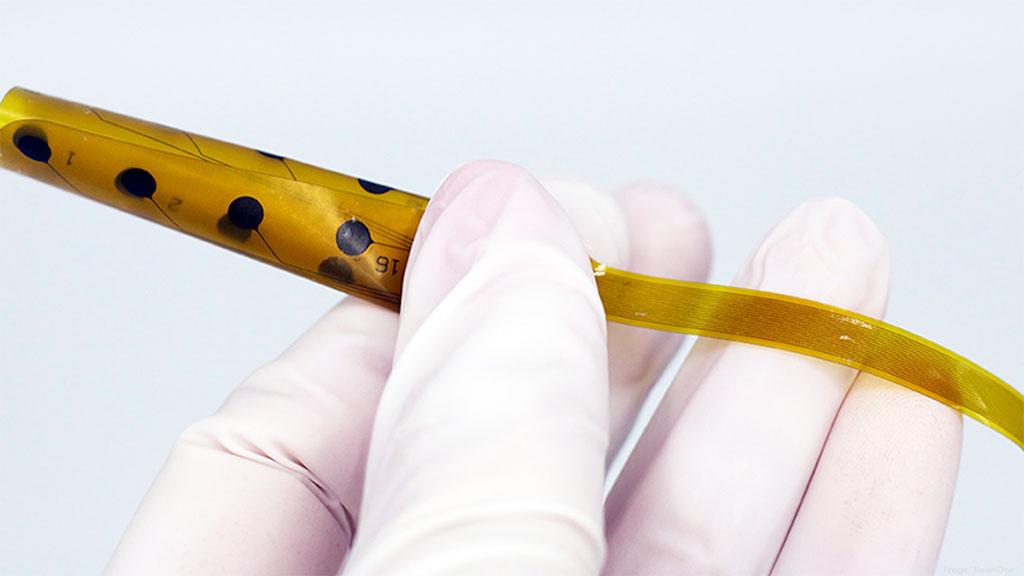
The rolled-up Evo Thin Film Electrode (Photo courtesy of NeuroOne Medical Technologies)
The single thin tail design allows the implanted electrode tail to be tunneled through one incision, helping to reduce infection risk and procedure time. In addition, the disposable cable assembly sent with each Evo sEEG Electrode removes the need to source the correct cables being used in surgery. As a result, hospital resources are freed from the management of sterilizing and storing individual electrode cable assemblies, and faster order fulfillment is made possible due to the automated manufacturing process.
“The next steps are to expand the sEEG labeling for longer term use, which we believe will be a key part of our commercialization strategy for the Evo sEEG electrode, and complete development of an sEEG ablation electrode/probe for ablation of brain tissue and permanently implanted stimulation electrodes for patients with chronic applications such as epilepsy, Parkinson's disease, and chronic back pain,” said Dave Rosa, CEO of NeuroOne Medical Technologies.
sEEG was first developed for invasive mapping of refractory focal epilepsy and usually involves implanting deep electrodes. But compared with grid placements, sEEG is less invasive and offers precise recordings from cortical and subcortical structures without using large craniotomies. Postoperative complications are rare, and usually include subdural, epidural, and intraparenchymal hemorrhage.
Related Links:
NeuroOne Medical Technologies














