Cancer “Flashlight” Shows Who Can Benefit from Targeted Treatments
Posted on 14 Jan 2026
Targeted cancer therapies can be highly effective, but only when a patient’s tumor expresses the specific protein the treatment is designed to attack. Determining this usually requires biopsies or advanced imaging, which can be invasive, time-consuming, and provide limited molecular detail. Researchers have now demonstrated a noninvasive imaging approach that can rapidly identify tumors expressing a key cancer-associated protein and help determine which patients are most likely to benefit from targeted therapies.
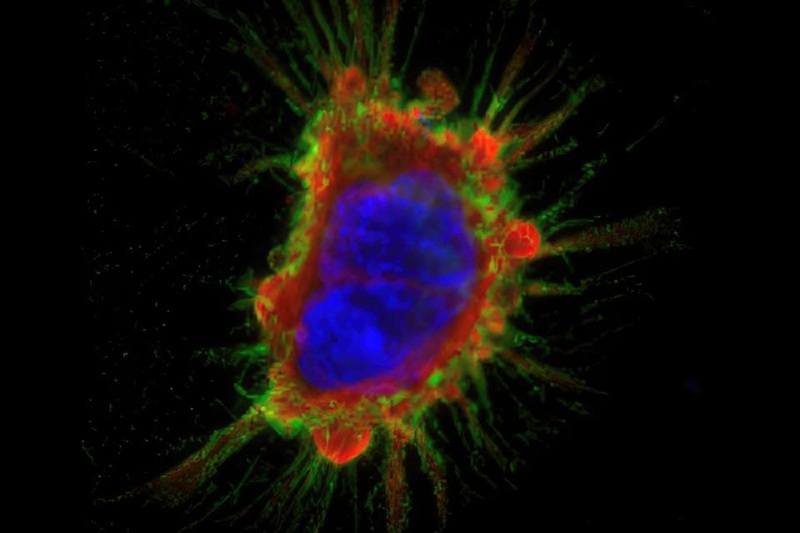
Researchers at the University of Missouri (Columbia, MO, USA) have developed a miniature antibody engineered to selectively bind to EphA2, a protein frequently overexpressed in many solid tumors and a growing target for cancer therapies. The antibody was labeled with a radioactive tracer, enabling it to be visualized using positron emission tomography (PET). Once injected, the radiolabeled antibody circulates through the body and binds to EphA2-positive tumor cells, causing these tumors to “light up” on PET scans within hours.

The approach was tested in mouse models bearing tumors with varying levels of EphA2 expression. PET imaging showed strong, selective signal accumulation in EphA2-positive tumors, while minimal signal was detected in healthy tissues or EphA2-negative tumors. This demonstrated that the imaging agent could accurately distinguish tumors based on their molecular profile. The findings were published in Molecular Imaging and Biology, validating the technique as a potential diagnostic tool for identifying patients suitable for EphA2-targeted treatments.
This imaging strategy could help clinicians rapidly assess whether a patient’s tumor is likely to respond to emerging EphA2-targeted therapies, avoiding ineffective treatments and unnecessary side effects. Because the method is noninvasive and delivers results much faster than biopsies, it may be especially valuable for patients who must travel long distances for care. The research team plans to further refine the technology and advance it from preclinical models toward human clinical trials over the coming years, to integrate it into precision oncology workflows.
“This new targeted approach is noninvasive, and you can get results from the imaging in hours rather than days, which can be huge for patients traveling long distances to seek treatment,” said Associate Professor Barry Edwards, lead author of the study. “By making the process easier and faster for both patients and clinicians, we’re showing that precision medicine is a win-win.”
Related Links:
University of Missouri